VEKLURY is administered via intravenous infusion1
Recommended dosing
Adult and pediatric patients weighing ≥40 kg should receive:
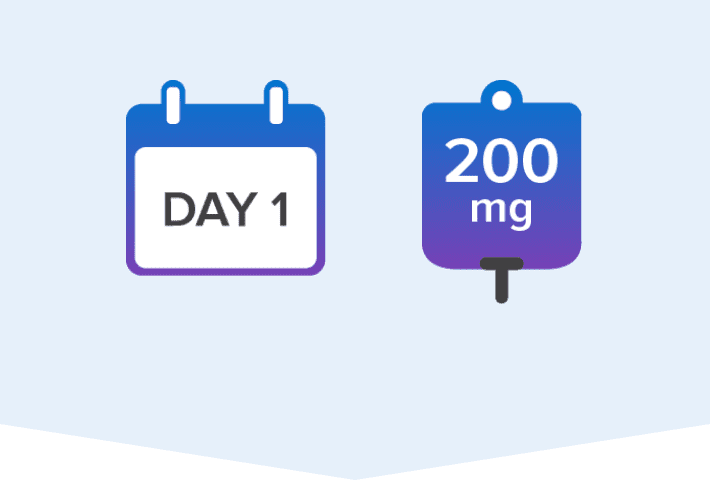
Loading dose of 200 mg IV

Once-daily maintenance dose of 100 mg IV
Pediatric patients ≥28 days old and weighing ≥3 kg to <40 kg should receive:
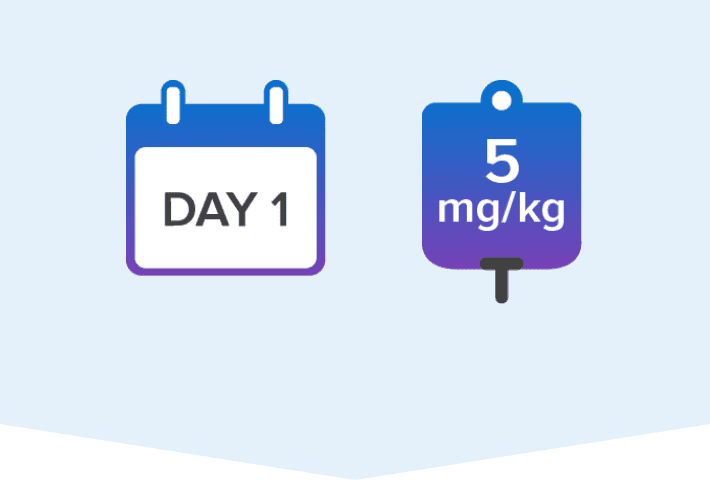
Loading dose of 5 mg/kg IV

Once-daily maintenance dose of 2.5 mg/kg IV
Pediatric patients (including term* neonates and infants) from birth to <28 days old and weighing ≥1.5 kg, and pediatric patients ≥28 days old and weighing 1.5 kg to <3 kg should receive:

Loading dose of 2.5 mg/kg IV

Once-daily maintenance dose of 1.25 mg/kg IV
For pediatric patients weighing ≥1.5 kg to <40 kg, a small 0.9% sodium chloride injection infusion bag (eg, 25 mL, 50 mL, or 100 mL) or an appropriately sized syringe should be used for pediatric dosing. A syringe and syringe pump may be used for infusion volumes <50 mL.
Recommended total treatment duration

Flexible infusion time between 30 and 120 minutes

For patients who are NOT hospitalized, have mild-to-moderate COVID-19, and are at high risk for progression to severe COVID-19, including hospitalization or death

For hospitalized patients NOT requiring invasive mechanical ventilation and/or ECMO
If a patient does not demonstrate clinical improvement, treatment may be extended for up to 5 additional days for a total treatment duration of up to 10 days
For hospitalized patients requiring invasive mechanical ventilation and/or ECMO
VEKLURY is indicated for the treatment of COVID-19 in adults and pediatric patients (birth to <18 years of age weighing ≥1.5 kg), who are:
- Hospitalized, or
- Not hospitalized, have mild-to-moderate COVID-19, and are at high risk for progression to severe COVID-19, including hospitalization or death.
VEKLURY is the only FDA-approved, COVID-19 antiviral1:

With no DDI-related contraindications and few expected clinically significant drug interactions.
- There is a risk of reduced antiviral activity when coadministered with chloroquine or hydroxychloroquine. Due to potential antagonism observed in vitro, concomitant use of VEKLURY with chloroquine phosphate or hydroxychloroquine sulfate is not recommended
Important Dosage and Administration Safety Information
Dosage and administration
- Administration should take place under conditions where management of severe hypersensitivity reactions, such as anaphylaxis, is possible.
- Treatment duration:
- For patients who are hospitalized, VEKLURY should be initiated as soon as possible after diagnosis of symptomatic COVID-19.
- For patients who are hospitalized and do not require invasive mechanical ventilation and/or ECMO, the recommended treatment duration is 5 days. If a patient does not demonstrate clinical improvement, treatment may be extended up to 5 additional days, for a total treatment duration of up to 10 days.
- For patients who are hospitalized and require invasive mechanical ventilation and/or ECMO, the recommended total treatment duration is 10 days.
- For patients who are not hospitalized, diagnosed with mild-to-moderate COVID-19, and are at high risk for progression to severe COVID-19, including hospitalization or death, the recommended total treatment duration is 3 days. VEKLURY should be initiated as soon as possible after diagnosis of symptomatic COVID-19 and within 7 days of symptom onset for outpatient use.
- Testing prior to and during treatment: Perform hepatic laboratory and prothrombin time testing prior to initiating VEKLURY and during use as clinically appropriate.
- Renal impairment: No dosage adjustment of VEKLURY is recommended in patients with any degree of renal impairment, including patients on dialysis. VEKLURY may be administered without regard to the timing of dialysis.
Product appearance

- VEKLURY for injection, 100 mg, is a sterile, preservative-free, white to off-white to yellow lyophilized powder—color does not affect product stability—in a single-dose, clear glass vial
- Red cap on vial
Dose preparation

- VEKLURY for injection, 100 mg, lyophilized powder, must be reconstituted with 19 mL Sterile Water for Injection and diluted in a 100 mL or 250 mL 0.9% sodium chloride infusion bag prior to administration
- For pediatric patients (birth to <18 years of age) weighing ≥1.5 kg to <40 kg, the 100 mg/20 mL (5 mg/mL) reconstituted solution should be further diluted to a fixed concentration of 1.25 mg/mL using 0.9% sodium chloride injection
Reconstitution
- To reconstitute VEKLURY for injection, lyophilized powder, add 19 mL of Sterile Water for Injection using a suitably sized syringe and needle per vial
- Following reconstitution, the vial will contain 100 mg/20 mL (5 mg/mL) of VEKLURY solution
Dilution
- Dilute reconstituted product immediately in either a 100 mL or 250 mL 0.9% sodium chloride infusion bag
- For pediatric patients (birth to <18 years of age) weighing ≥1.5 kg to <40 kg, the 100 mg/20 mL (5 mg/mL) reconstituted solution should be further diluted to a fixed concentration of 1.25 mg/mL using 0.9% sodium chloride injection
- Small 0.9% sodium chloride injection infusion bags or an appropriately sized syringe should be used for pediatric dosing
- A syringe and syringe pump may be used for infusion volumes <50 mL
- It is always recommended to administer IV medication immediately after preparation when possible
- The prepared infusion solution in the infusion bag is stable for 24 hours at room temperature (20 °C to 25 °C [68 °F to 77 °F]) or 48 hours at refrigerated temperature (2 °C to 8 °C [36 °F to 46 °F])
- The prepared infusion syringe should be used immediately
Administration in adult and pediatric patients weighing ≥40 kg
| Infusion bag volume | Infusion time | Rate of infusion |
|---|---|---|
| 250 mL | 30 minutes | 8.33 mL/min |
| 60 minutes | 4.17 mL/min | |
| 120 minutes | 2.08 mL/min | |
| 100 mL | 30 minutes | 3.33 mL/min |
| 60 minutes | 1.67 mL/min | |
| 120 minutes | 0.83 mL/min |
For pediatric patients weighing ≥1.5 kg to <40 kg, administer VEKLURY via intravenous infusion over 30 to 120 minutes, the rate (mL/min) of which should be calculated based on the total infusion volume and total infusion time.
How is VEKLURY supplied?

- VEKLURY for injection, 100 mg, is supplied as a single-dose vial containing a sterile, preservative-free lyophilized powder. It requires reconstitution and further dilution prior to administration by intravenous infusion
- Unopened vials of VEKLURY lyophilized powder can be stored below 30 °C (86 °F)
- Once opened, do not reuse reconstituted or diluted VEKLURY infusion solution
- VEKLURY does not contain any preservatives. Care should be taken to prevent inadvertent microbial contamination. Any unused portion of a single-dose VEKLURY vial should be discarded after a diluted solution is prepared
Please see additional information on the storage, handling, preparation, and administration of VEKLURY in the full Prescribing Information.
Important Safety Information
Contraindication
- VEKLURY is contraindicated in patients with a history of clinically significant hypersensitivity reactions to VEKLURY or any of its components.
Warnings and precautions
- Hypersensitivity, including infusion-related and anaphylactic reactions: Hypersensitivity, including infusion-related and anaphylactic reactions, has been observed during and following administration of VEKLURY; most reactions occurred within 1 hour. Monitor patients during infusion and observe for at least 1 hour after infusion is complete for signs and symptoms of hypersensitivity as clinically appropriate. Symptoms may include hypotension, hypertension, tachycardia, bradycardia, hypoxia, fever, dyspnea, wheezing, angioedema, rash, nausea, diaphoresis, and shivering. Slower infusion rates (maximum infusion time of up to 120 minutes) can potentially prevent these reactions. If a severe infusion-related hypersensitivity reaction occurs, immediately discontinue VEKLURY and initiate appropriate treatment (see Contraindications).
- Increased risk of transaminase elevations: Transaminase elevations have been observed in healthy volunteers and in patients with COVID-19 who received VEKLURY; these elevations have also been reported as a clinical feature of COVID-19. Perform hepatic laboratory testing in all patients (see Dosage and administration). Consider discontinuing VEKLURY if ALT levels increase to >10x ULN. Discontinue VEKLURY if ALT elevation is accompanied by signs or symptoms of liver inflammation.
- Risk of reduced antiviral activity when coadministered with chloroquine or hydroxychloroquine: Coadministration of VEKLURY with chloroquine phosphate or hydroxychloroquine sulfate is not recommended based on data from cell culture experiments, demonstrating potential antagonism, which may lead to a decrease in the antiviral activity of VEKLURY.
Adverse reactions
- The most common adverse reaction (≥5% all grades) was nausea.
- The most common lab abnormalities (≥5% all grades) were increases in ALT and AST.
Dosage and administration
- Administration should take place under conditions where management of severe hypersensitivity reactions, such as anaphylaxis, is possible.
- Treatment duration:
- For patients who are hospitalized, VEKLURY should be initiated as soon as possible after diagnosis of symptomatic COVID-19.
- For patients who are hospitalized and do not require invasive mechanical ventilation and/or ECMO, the recommended treatment duration is 5 days. If a patient does not demonstrate clinical improvement, treatment may be extended up to 5 additional days, for a total treatment duration of up to 10 days.
- For patients who are hospitalized and require invasive mechanical ventilation and/or ECMO, the recommended total treatment duration is 10 days.
- For patients who are not hospitalized, diagnosed with mild-to-moderate COVID-19, and are at high risk for progression to severe COVID-19, including hospitalization or death, the recommended total treatment duration is 3 days. VEKLURY should be initiated as soon as possible after diagnosis of symptomatic COVID-19 and within 7 days of symptom onset for outpatient use.
- Testing prior to and during treatment: Perform hepatic laboratory and prothrombin time testing prior to initiating VEKLURY and during use as clinically appropriate.
- Renal impairment: No dosage adjustment of VEKLURY is recommended in patients with any degree of renal impairment, including patients on dialysis. VEKLURY may be administered without regard to the timing of dialysis.
Pregnancy and lactation
- Pregnancy: A pregnancy registry has been established for VEKLURY. Available clinical trial data for VEKLURY in pregnant women have not identified a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes following second- and third-trimester exposure. There are insufficient data to evaluate the risk of VEKLURY exposure during the first trimester. Maternal and fetal risks are associated with untreated COVID-19 in pregnancy.
- Lactation: VEKLURY can pass into breast milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for VEKLURY and any potential adverse effects on the breastfed child from VEKLURY or from an underlying maternal condition. Breastfeeding individuals with COVID-19 should follow practices according to clinical guidelines to avoid exposing the infant to COVID-19.
INDICATION
VEKLURY is indicated for the treatment of COVID-19 in adults and pediatric patients (birth to <18 years of age weighing ≥1.5 kg), who are:
- Hospitalized, or
- Not hospitalized, have mild-to-moderate COVID-19, and are at high risk for progression to severe COVID-19, including hospitalization or death.
Please see full Prescribing Information for VEKLURY.
*Gestational age >37 weeks.
DDI=drug-drug interaction; ECMO=extracorporeal membrane oxygenation.

