VEKLURY improved clinical outcomes in hospitalized patients with severe COVID-191-3
5 days of treatment with VEKLURY led to similar clinical outcomes as a 10-day course1,2
OVER 50%
of patients saw clinical improvements of ≥2 points at Day 14 with VEKLURY
65% in the 5-day arm and 54% in the 10-day arm; odds ratio for improvement: 0.75 (95% CI, 0.51 to 1.12)*
*Once adjusted for between-group differences at baseline.
OVER 50%
of patients experienced recovery at Day 14 with VEKLURY
64% in the 5-day arm and 54% in the 10-day arm; baseline-adjusted difference in proportion: -6.3% (95% CI, -15.4 to 2.8)
- Recovery was defined as those with a baseline ordinal score of 2 to 5 who improved to a score of 6 or 7
All-cause mortality at Day 28
- No statistically significant differences between the 2 groups; 12% vs 14% in the 5- and 10-day treatment groups, respectively
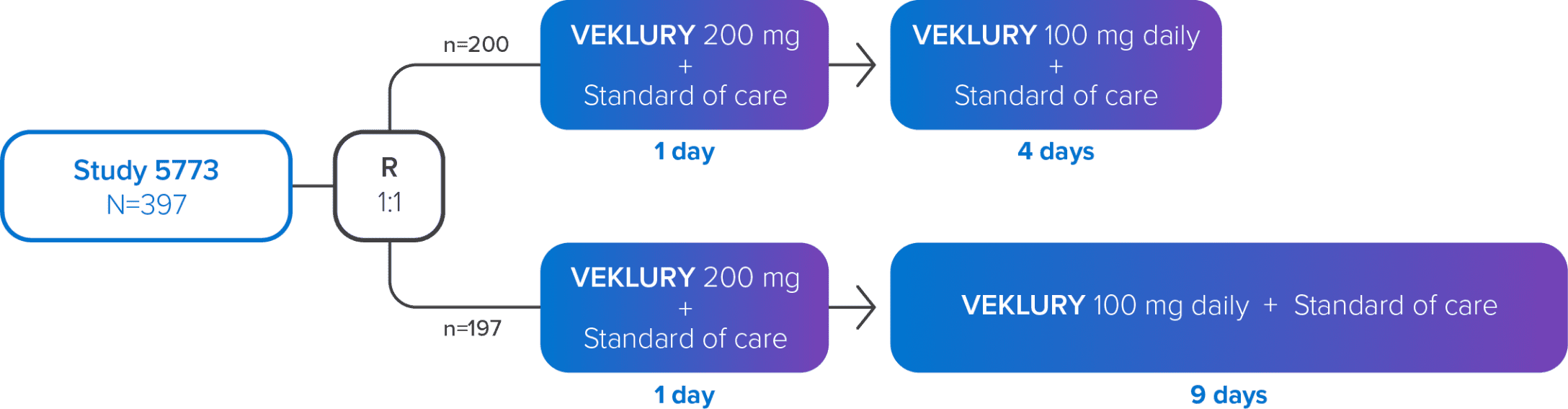
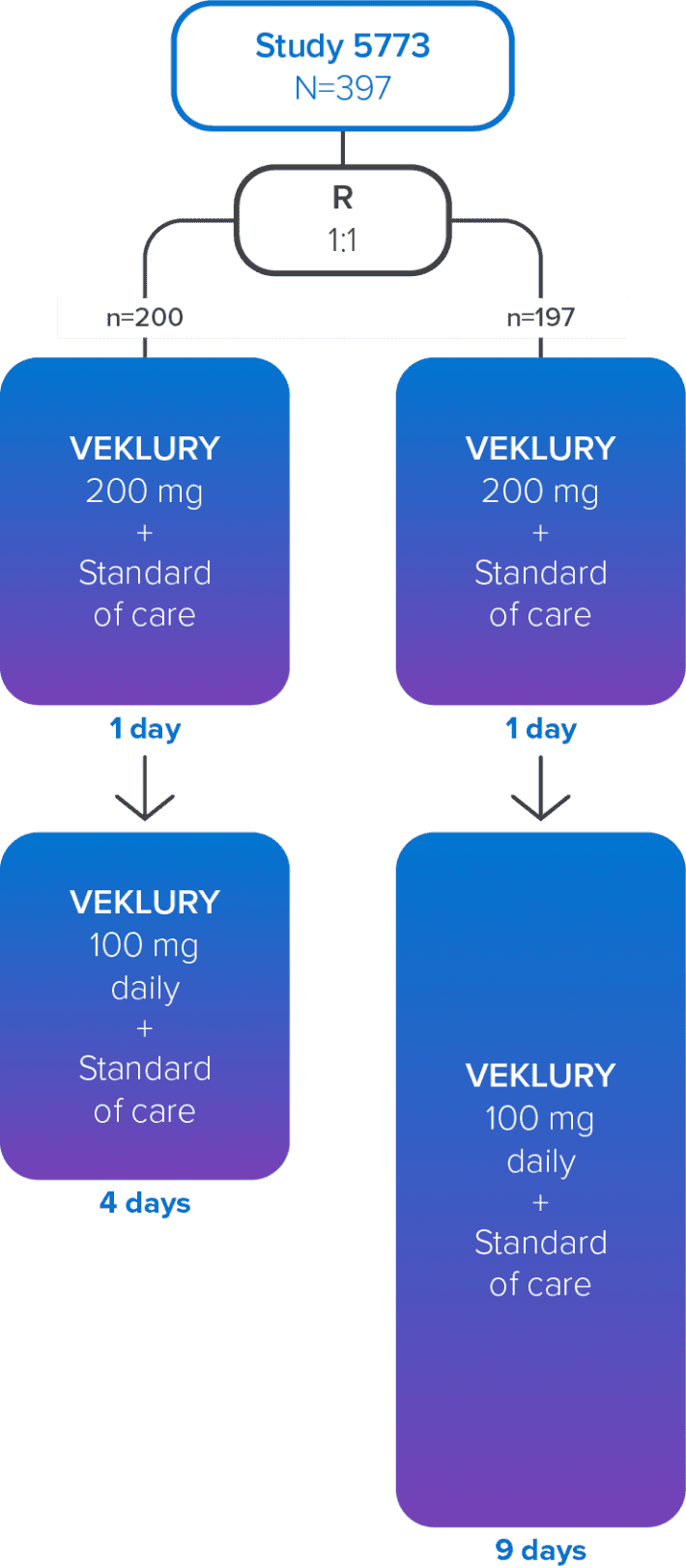
Study GS-US-540-5773 was a randomized, open-label, multicenter, phase 3 study in hospitalized adult patients with confirmed SARS-CoV-2 infection, an SpO2 of ≤94% on room air or receiving supplemental oxygen, and radiological evidence of pneumonia.1,2
Study design1,2
Treatment with VEKLURY was stopped in patients who were discharged from the hospital prior to completion of their protocol-defined duration of treatment. Patients on mechanical ventilation at screening were excluded.
Primary endpoint
Clinical status on Day 14 as assessed on a 7-point ordinal scale.
R=randomization.
Safety parameters in Study 57731
VEKLURY had a similar safety profile in 5- and 10-day treatment courses
Adverse reactions were similar in both VEKLURY study arms
The most common adverse reactions occurring in ≥5% of patients in either the VEKLURY 5-day or 10-day group, respectively, were nausea (5% vs 3%), AST increased (3% vs 6%), and ALT increased (2% vs 7%).
| Types of adverse reactions | VEKLURY 5 days(n=200)n (%) | VEKLURY 10 days(n=197)n (%) |
|---|---|---|
| Any adverse reaction, all Grades | 33 (17) | 40 (20) |
| Serious adverse reactions | 3 (2)* | 4 (2)* |
| Adverse reactions leading to treatment discontinuation | 5 (3)† | 9 (5)† |
*Transaminases increased (n=5), hepatic enzyme increased (n=1), hypertransaminasemia (n=1).
†Transaminases increased (n=4), hepatic enzyme increased (n=2), LFT increased (n=2), hypertransaminasemia (n=1), ALT increased (n=1), ALT increased and AST increased (n=2), injection site erythema (n=1), rash (n=1).
Laboratory abnormalities (Grades 3–4) reported in ≥3% of patients
| Laboratory parameter abnormality‡ | VEKLURY 5 days(n=200) | VEKLURY 10 days(n=197) |
|---|---|---|
| ALT increased | 6% | 8% |
| AST increased | 7% | 6% |
| Creatinine clearance decreased§ | 10% | 19% |
| Creatinine increased | 5% | 15% |
| Glucose increased | 11% | 8% |
| Hemoglobin decreased | 6% | 8% |
‡Frequencies are based on treatment-emergent laboratory abnormalities graded per Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events, Version 2.1, dated July 2017.
§Based on the Cockcroft-Gault formula.
Baseline characteristics2
| Characteristic | 5-day group(n=200) | 10-day group(n=197) |
|---|---|---|
| Median age (IQR), y | 61 (50-69) | 62 (50-71) |
| Male sex | 60% | 68% |
| Race | ||
| White | 71% | 70% |
| Black | 10% | 12% |
| Asian | 10% | 13% |
| Respiratory Status | ||
| Low-flow oxygen | 56% | 54% |
| High-flow oxygen | 24% | 30% |
| Invasive mechanical ventilation/ECMO | 2% | 5% |
| Coexisting conditions of interest | ||
| Diabetes | 24% | 22% |
| Hyperlipidemia | 20% | 25% |
| Hypertension | 50% | 50% |
| Asthma | 14% | 11% |
ECMO=extracorporeal membrane oxygenation; IQR=interquartile range.
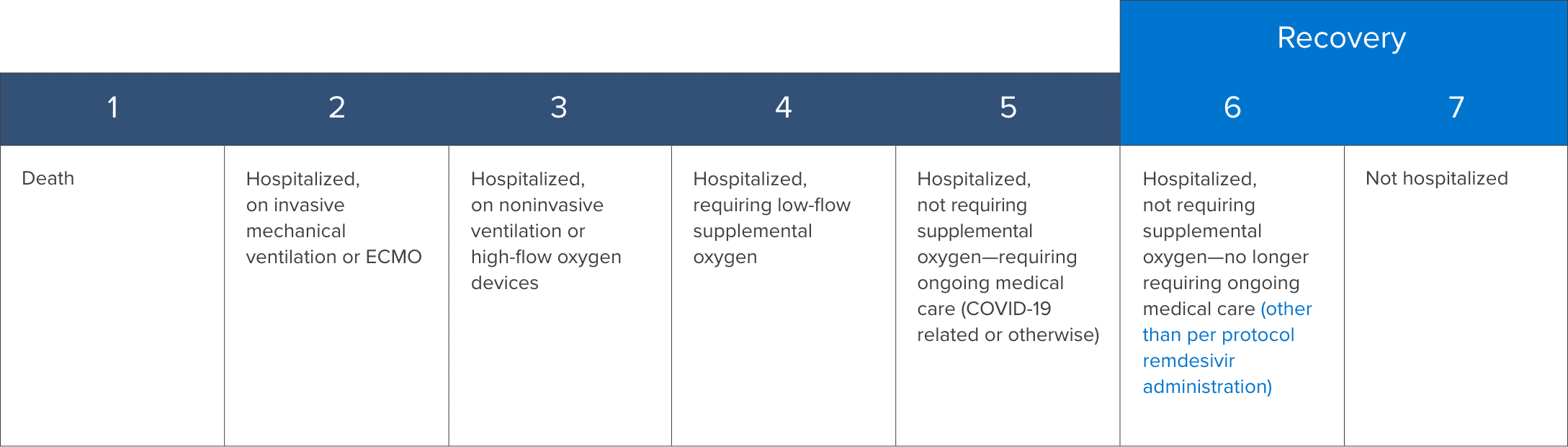
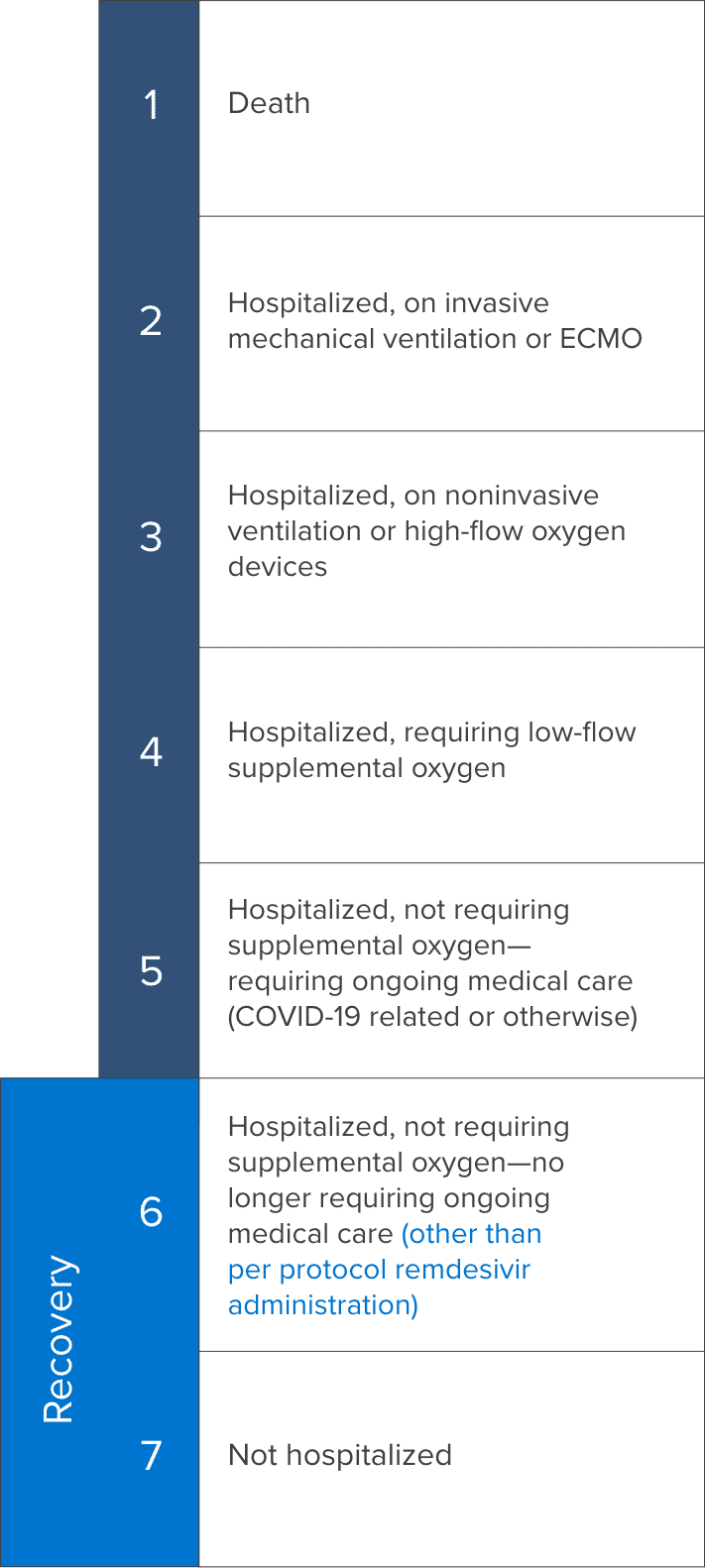
7-point ordinal scale1,3
Patient clinical status was assessed on a 7-point ordinal scale with a lower score indicating greater clinical severity.
ECMO=extracorporeal membrane oxygenation.
See VEKLURY data on disease progression and recovery time
ACTT-1 STUDYSee improved clinical outcomes from earlier use of VEKLURY
STUDY 5774Important Safety Information
Collapse
Contraindication
- VEKLURY is contraindicated in patients with a history of clinically significant hypersensitivity reactions to VEKLURY or any of its components.
Warnings and precautions
- Hypersensitivity, including infusion-related and anaphylactic reactions: Hypersensitivity, including infusion-related and anaphylactic reactions, has been observed during and following administration of VEKLURY; most reactions occurred within 1 hour. Monitor patients during infusion and observe for at least 1 hour after infusion is complete for signs and symptoms of hypersensitivity as clinically appropriate. Symptoms may include hypotension, hypertension, tachycardia, bradycardia, hypoxia, fever, dyspnea, wheezing, angioedema, rash, nausea, diaphoresis, and shivering. Slower infusion rates (maximum infusion time of up to 120 minutes) can potentially prevent these reactions. If a severe infusion-related hypersensitivity reaction occurs, immediately discontinue VEKLURY and initiate appropriate treatment (see Contraindications).
- Increased risk of transaminase elevations: Transaminase elevations have been observed in healthy volunteers and in patients with COVID-19 who received VEKLURY; these elevations have also been reported as a clinical feature of COVID-19. Perform hepatic laboratory testing in all patients (see Dosage and administration). Consider discontinuing VEKLURY if ALT levels increase to >10x ULN. Discontinue VEKLURY if ALT elevation is accompanied by signs or symptoms of liver inflammation.
- Risk of reduced antiviral activity when coadministered with chloroquine or hydroxychloroquine: Coadministration of VEKLURY with chloroquine phosphate or hydroxychloroquine sulfate is not recommended based on data from cell culture experiments, demonstrating potential antagonism, which may lead to a decrease in the antiviral activity of VEKLURY.
Adverse reactions
- The most common adverse reaction (≥5% all grades) was nausea.
- The most common lab abnormalities (≥5% all grades) were increases in ALT and AST.
Dosage and administration
- Administration should take place under conditions where management of severe hypersensitivity reactions, such as anaphylaxis, is possible.
- Treatment duration:
- For patients who are hospitalized, VEKLURY should be initiated as soon as possible after diagnosis of symptomatic COVID-19.
- For patients who are hospitalized and do not require invasive mechanical ventilation and/or ECMO, the recommended treatment duration is 5 days. If a patient does not demonstrate clinical improvement, treatment may be extended up to 5 additional days, for a total treatment duration of up to 10 days.
- For patients who are hospitalized and require invasive mechanical ventilation and/or ECMO, the recommended total treatment duration is 10 days.
- For patients who are not hospitalized, diagnosed with mild-to-moderate COVID-19, and are at high risk for progression to severe COVID-19, including hospitalization or death, the recommended total treatment duration is 3 days. VEKLURY should be initiated as soon as possible after diagnosis of symptomatic COVID-19 and within 7 days of symptom onset for outpatient use.
- Testing prior to and during treatment: Perform hepatic laboratory and prothrombin time testing prior to initiating VEKLURY and during use as clinically appropriate.
- Renal impairment: No dosage adjustment of VEKLURY is recommended in patients with any degree of renal impairment, including patients on dialysis. VEKLURY may be administered without regard to the timing of dialysis.
Pregnancy and lactation
- Pregnancy: A pregnancy registry has been established for VEKLURY. Available clinical trial data for VEKLURY in pregnant women have not identified a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes following second- and third-trimester exposure. There are insufficient data to evaluate the risk of VEKLURY exposure during the first trimester. Maternal and fetal risks are associated with untreated COVID-19 in pregnancy.
- Lactation: VEKLURY can pass into breast milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for VEKLURY and any potential adverse effects on the breastfed child from VEKLURY or from an underlying maternal condition. Breastfeeding individuals with COVID-19 should follow practices according to clinical guidelines to avoid exposing the infant to COVID-19.
INDICATION
View All
Collapse
VEKLURY is indicated for the treatment of COVID-19 in adults and pediatric patients (birth to <18 years of age weighing ≥1.5 kg), who are:
VEKLURY is indicated for the treatment of COVID-19 in adults and pediatric patients (birth to <18 years of age weighing ≥1.5 kg), who are:
Please see full Prescribing Information for VEKLURY.
LFT=liver function test; SpO2=oxygen saturation.
View All
INDICATION
View All
Collapse
VEKLURY is indicated for the treatment of COVID-19 in adults and pediatric patients (birth to <18 years of age weighing ≥1.5 kg), who are:
VEKLURY is indicated for the treatment of COVID-19 in adults and pediatric patients (birth to <18 years of age weighing ≥1.5 kg), who are:
Please see full Prescribing Information for VEKLURY.
Important Safety Information
Collapse
Contraindication
- VEKLURY is contraindicated in patients with a history of clinically significant hypersensitivity reactions to VEKLURY or any of its components.
Warnings and precautions
- Hypersensitivity, including infusion-related and anaphylactic reactions: Hypersensitivity, including infusion-related and anaphylactic reactions, has been observed during and following administration of VEKLURY; most reactions occurred within 1 hour. Monitor patients during infusion and observe for at least 1 hour after infusion is complete for signs and symptoms of hypersensitivity as clinically appropriate. Symptoms may include hypotension, hypertension, tachycardia, bradycardia, hypoxia, fever, dyspnea, wheezing, angioedema, rash, nausea, diaphoresis, and shivering. Slower infusion rates (maximum infusion time of up to 120 minutes) can potentially prevent these reactions. If a severe infusion-related hypersensitivity reaction occurs, immediately discontinue VEKLURY and initiate appropriate treatment (see Contraindications).
- Increased risk of transaminase elevations: Transaminase elevations have been observed in healthy volunteers and in patients with COVID-19 who received VEKLURY; these elevations have also been reported as a clinical feature of COVID-19. Perform hepatic laboratory testing in all patients (see Dosage and administration). Consider discontinuing VEKLURY if ALT levels increase to >10x ULN. Discontinue VEKLURY if ALT elevation is accompanied by signs or symptoms of liver inflammation.
- Risk of reduced antiviral activity when coadministered with chloroquine or hydroxychloroquine: Coadministration of VEKLURY with chloroquine phosphate or hydroxychloroquine sulfate is not recommended based on data from cell culture experiments, demonstrating potential antagonism, which may lead to a decrease in the antiviral activity of VEKLURY.
Adverse reactions
- The most common adverse reaction (≥5% all grades) was nausea.
- The most common lab abnormalities (≥5% all grades) were increases in ALT and AST.
Dosage and administration
- Administration should take place under conditions where management of severe hypersensitivity reactions, such as anaphylaxis, is possible.
- Treatment duration:
- For patients who are hospitalized, VEKLURY should be initiated as soon as possible after diagnosis of symptomatic COVID-19.
- For patients who are hospitalized and do not require invasive mechanical ventilation and/or ECMO, the recommended treatment duration is 5 days. If a patient does not demonstrate clinical improvement, treatment may be extended up to 5 additional days, for a total treatment duration of up to 10 days.
- For patients who are hospitalized and require invasive mechanical ventilation and/or ECMO, the recommended total treatment duration is 10 days.
- For patients who are not hospitalized, diagnosed with mild-to-moderate COVID-19, and are at high risk for progression to severe COVID-19, including hospitalization or death, the recommended total treatment duration is 3 days. VEKLURY should be initiated as soon as possible after diagnosis of symptomatic COVID-19 and within 7 days of symptom onset for outpatient use.
- Testing prior to and during treatment: Perform hepatic laboratory and prothrombin time testing prior to initiating VEKLURY and during use as clinically appropriate.
- Renal impairment: No dosage adjustment of VEKLURY is recommended in patients with any degree of renal impairment, including patients on dialysis. VEKLURY may be administered without regard to the timing of dialysis.
Pregnancy and lactation
- Pregnancy: A pregnancy registry has been established for VEKLURY. Available clinical trial data for VEKLURY in pregnant women have not identified a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes following second- and third-trimester exposure. There are insufficient data to evaluate the risk of VEKLURY exposure during the first trimester. Maternal and fetal risks are associated with untreated COVID-19 in pregnancy.
- Lactation: VEKLURY can pass into breast milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for VEKLURY and any potential adverse effects on the breastfed child from VEKLURY or from an underlying maternal condition. Breastfeeding individuals with COVID-19 should follow practices according to clinical guidelines to avoid exposing the infant to COVID-19.

-Logo-KO-Blue-Image.svg)